Water properties¶
Seawater properties (especially water density) are important in hydrology, e.g., for salt intrusion. The implemented water properties are based on Algorithms for computation of fundamental properties of seawater. Unesco technical papers in marine science 44. Unesco 1983 (https://unesdoc.unesco.org/90e8e061-800c-4c51-b08d-3529216aaee4).
Pure water density¶
The density of water at zero relative pressure (one atmosphere or 101325 Pa) and zero salinity as a function of temperature can be calculated according to different methods. According to Craig (eq. 14):
where:
t = temperature in °C\(a_0\) = 999.842594\(a_1\) = 6.793952E-2\(a_2\) = -9.095290E-3\(a_3\) = 1.001685E-4\(a_4\) = -1.120083E-6\(a_5\) = 6.536332E-9
print(pure_water_density_craig(20))
Output:
998.2063193824
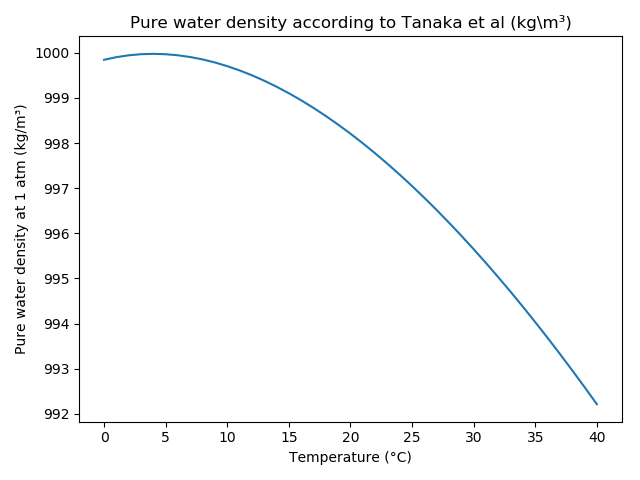
Pure water density according to Tanaka, M., et. al; Recommended table for the density of water between 0°C and 40°C based on recent experimental reports, Metrologia, 2001, 38, 301-309 (http://www.personal.psu.edu/users/m/r/mrh318/water-properties/Tanaka-etal-M-2001.pdf) by:
where
t = temperature in °C\(a_1\) = -3.983035\(a_2\) = 301.797\(a_3\) = 522528.9\(a_4\) = 69.34881\(a_5\) = 999.974950
print(pure_water_density(20))
Output:
998.2067455596167
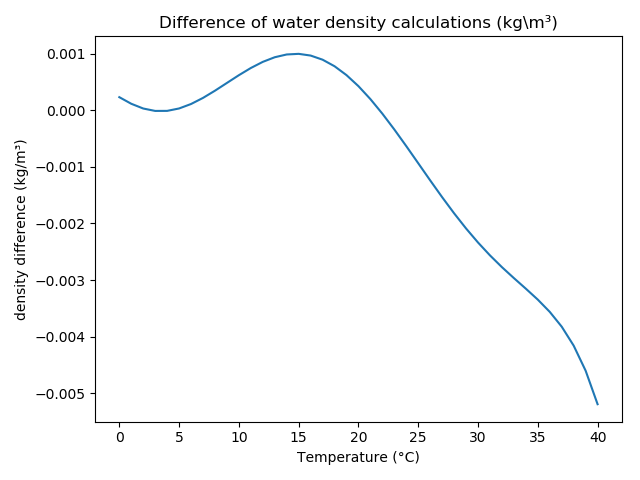
The following figures show the pure water density and the difference between the methods after Craig and Tanaka et al:
Water density depencency on salinity¶
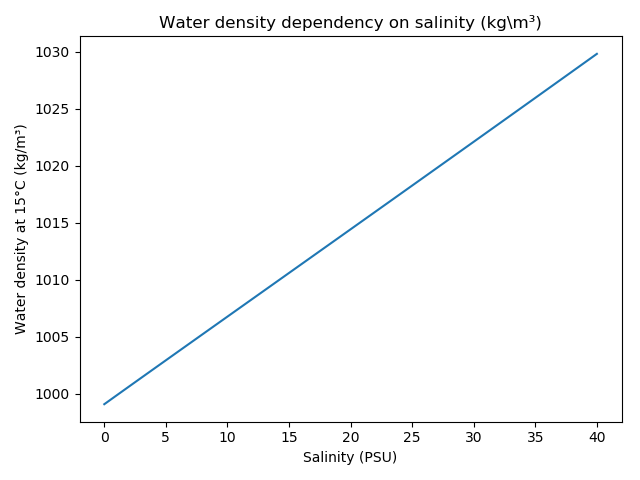
The density of water at zero relative pressure (one atmosphere or 101325 Pa) as a function of temperature and salinity can be calculated according to (eq. 13):
where
\(b0\) = 8.24493E-1\(b1\) = -4.0899E-3\(b2\) = 7.6438E-5\(b3\) = -8.2467E-7\(b4\) = 5.3875E-9\(c0\) = -5.72466E-3\(c1\) = 1.0227E-4\(c2\) = -1.6546E-6\(d0\) = 4.8314E-4
Water density¶
Water density is calculated by (eq. 7):
where
Secant bulk modulus of pure water¶
Secant bulk modulus at zero pressure¶
Secant bulk modulus of pure water¶
Water conductivity and salinity¶
Conductivity is usually expressed in micro- or millisiemens per centimeter (\(\mu S/cm\) or \(mS/cm\)). Typical drinking water has a conductivity between 0.05 and 0.50 mS/cm
Salinity is the total concentration of salts in water, usually expressed in g/kg. Seawater has a salinity of ca. 35 g/kg